Eosinophilic oesophagitis (EoE) is a chronic inflammatory disease of the oesophagus. This immune-mediated disorder is characterised clinically by oesophageal dysfunction and histologically by eosinophil-predominant inflammation. Common symptoms in adults with EoE include; dysphagia (difficulty swallowing) and odynophagia (pain with swallowing), in addition to chest pain or regurgitation (both occurring during or immediately after eating) and/or food bolus obstruction, the latter of which may require extraction in an emergency department.1,2 EoE was first described as a standalone clinicopathological only entity in 1993,3 however, the first consensus guidelines for diagnosis of EoE were not published until several years later, in 2007. These guidelines have now been updated a number of times since, including most recently by the British Society of Gastroenterology, in 2022.4,5
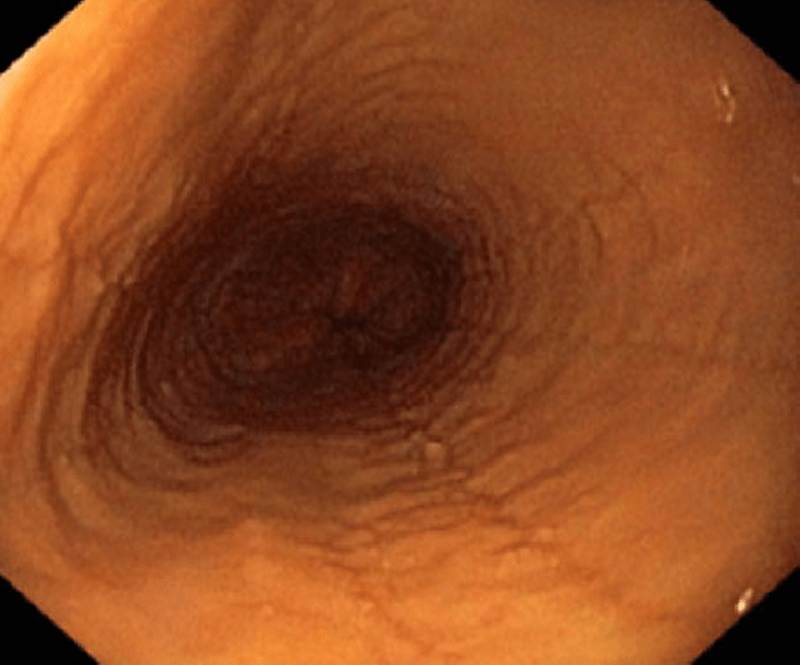
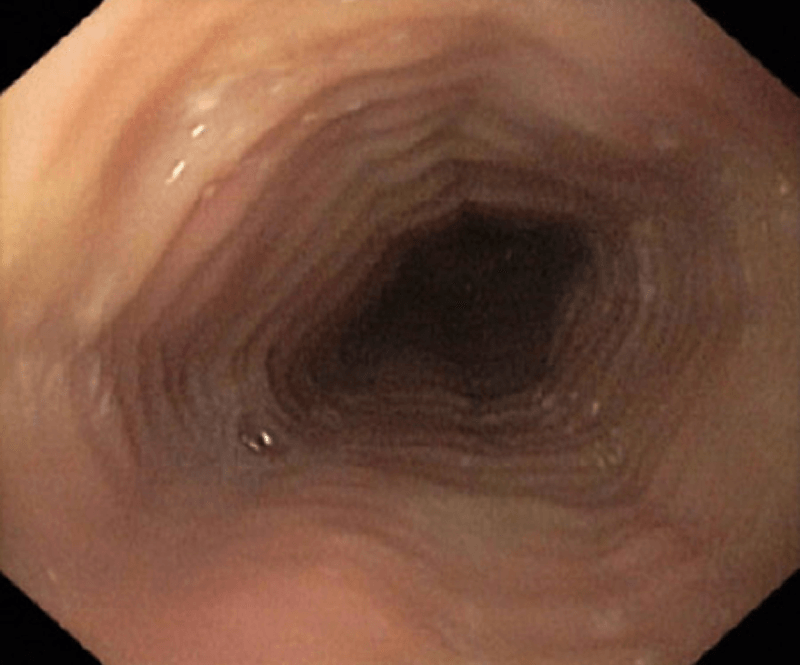
The aetiology of EoE is presumed to involve the ingestion of dietary &/or airborne substances, which in genetically susceptible individuals, are allergenic. In EoE, these allergens are presented to T helper (Th)-2 cells in the mucosa of oesophagus, eliciting the release of the cytokines; IL-4; -5 & -13, which with the chemokine, eotaxin-3, stimulate the maturation and migration of eosinophils to the oesophageal mucosa. There the eosinophils release pro-inflammatory and pro-fibrotic mediators, which, over time, lead to pathologic changes in the oesophageal mucosa, seen on biopsy (see Fig. 1). Over time, in a patient receiving no or ineffective treatment, this can lead to a fibrostenotic phenotype with a low calibre oesophagus and a high associated risk of recurrent food bolus obstructions requiring endoscopic rescue to remove the obstruction and the potential need for dilation procedures. Eventually, this leads to adverse macroscopic changes in the oesophageal wall, observed on endoscopy (see Fig. 2), associated with oesophageal dysmotility and the symptoms of oesophageal dysfunction. A history of atopic disorders, such as asthma, eczema or allergic rhinitis is common in EoE. However, it remains unclear whether atopy predisposes individuals to EoE.1,6–8 The most-relevant food allergens for EoE include animal milk and dairy products, wheat, eggs, soy, and nuts, as well as fish/shellfish and legumes.9 Aeroallergens and oral/sublingual immunotherapies have also been postulated as potential triggers of EoE.11,14
Adverse cellular and macroscope changes in EoE
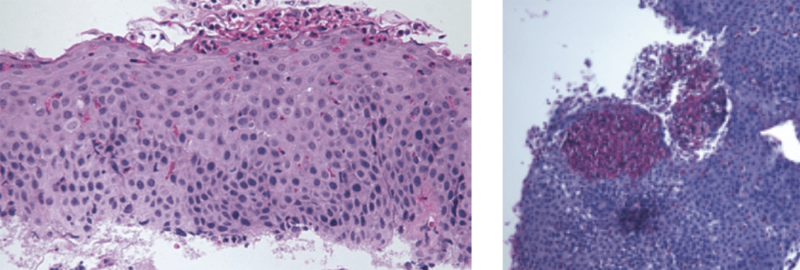
Furrows and oedema
Rings and oedema
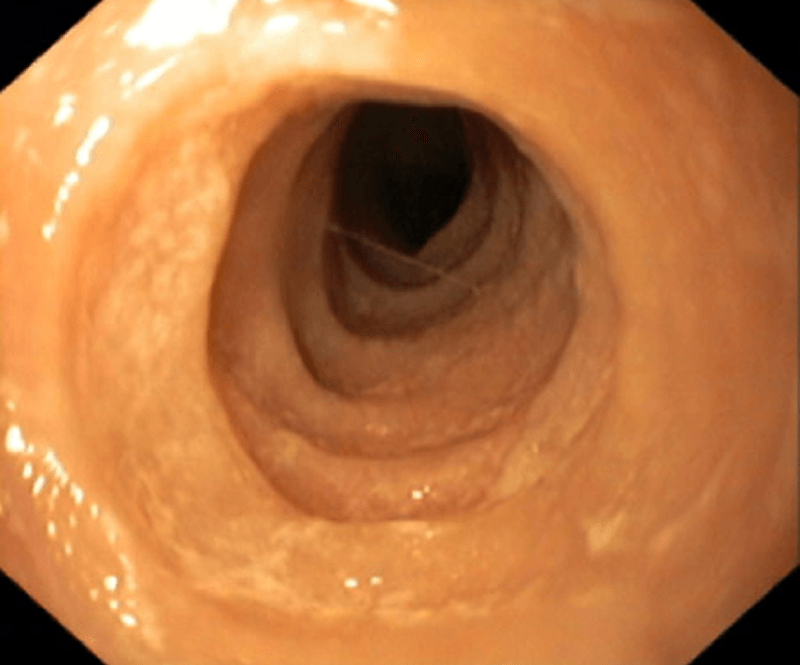
Rings (trachealisation), exudate, oedema
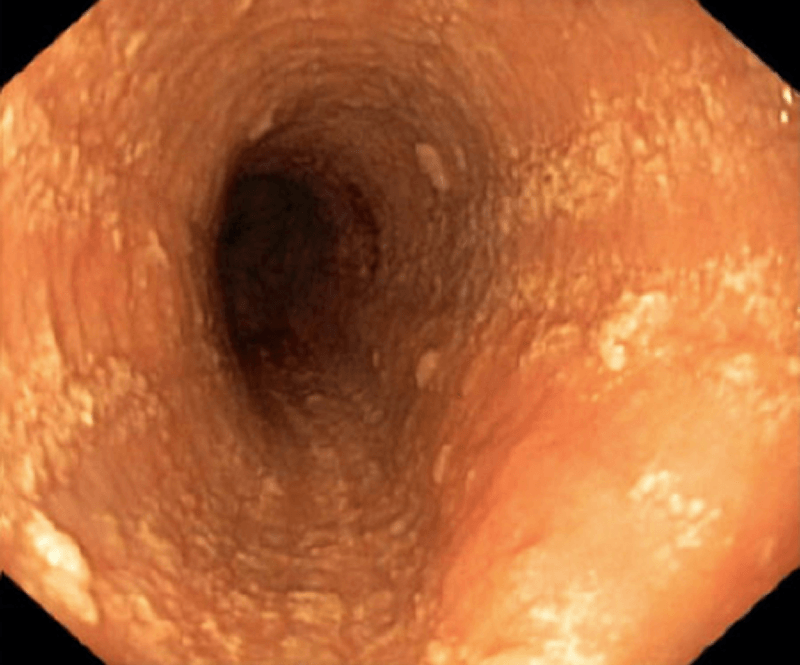
Exudate
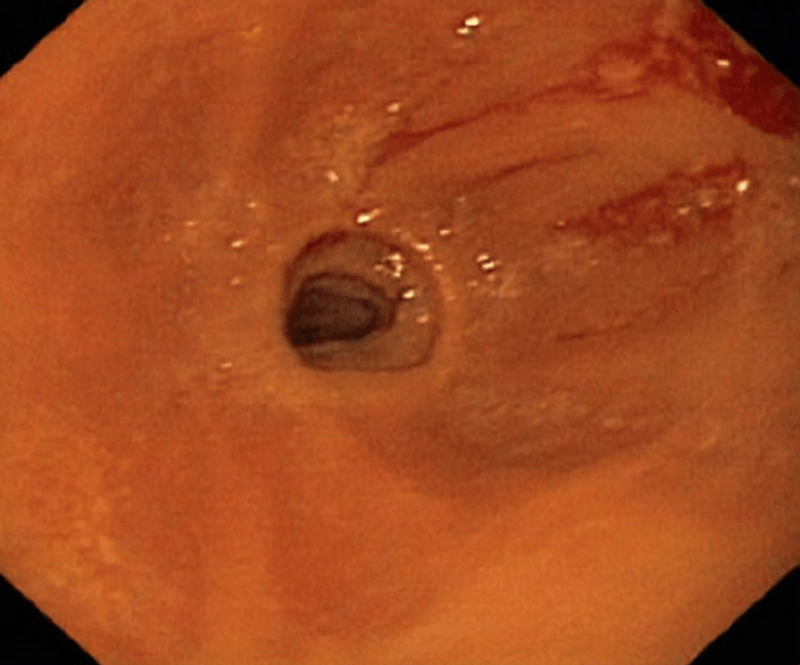
Strictures (severe)
Aetiology
There are a range of risk factors associated with EoE.8
| Aeroallergens | May cause EoE or increase disease activity; can cross react with food allergens; may explain seasonal variation in diagnosis |
| Food allergens | Directly trigger EoE; elimination can lead to disease remission |
| Helicobacter pylori | Inversely associated with EoE; decrease in H. pylori prevalence has accompanied increase in EoE prevalence over the last 20 years; aetiology unclear |
| Infections (herpes simplex virus; mycoplasma) | Associated with EoE; aetiology unclear |
| Oral or sublingual immunotherapy | Causes or induces EoE in certain patients; baseline EoE status for reported cases usually not know prior to immunotherapy |
| Proton pump inhibitors | Reported to induce IgE antibodies to certain foods |
| Cold or arid climates | Increased risk of EoE in these climate zones, but not in temperate or tropical zones |
| Population density | Risk of EoE increase as population density decreases |
| Early life factors | Antibiotic use, Cesarean section, and preterm delivery increase the odds of pediatric EoE |
| Connective tissue disorders | Ehlers-Danlos, Marfan Syndrome, and Loeys- Dietz syndrome have been associated with EoE |
| Coeliac disease | Associated with EoE; EoE is more common in patients with coeliac disease than would be expected |
| Autoimmune conditions | Inflammatory bowel disease, rheumatoid arthritis, IgA deficiency, multiple sclerosis, and Hashimoto’s thyroiditis associated with EoE |

EoE is the manifestation of a TH2 immune response involving activated eosinophils, mast cells and the cytokines eotaxin-3, interleukin-5, and interleukin-13. Genetic susceptibility factors (TSLP, CAPN14) have also been reported.12
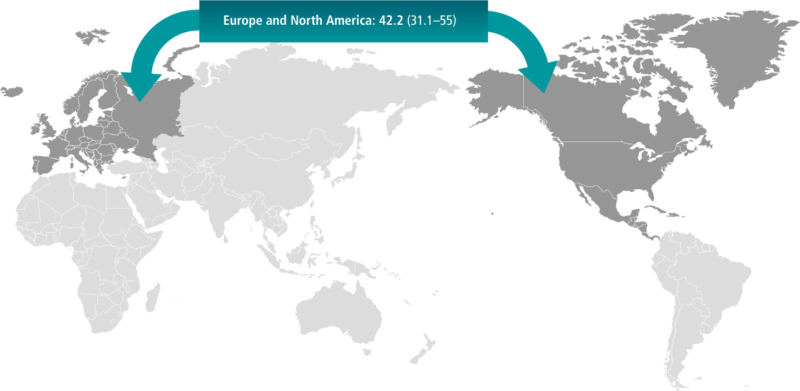
EoE is the most most frequent eosinophilic gastrointestinal disorder as well as the second most common cause of dysphagia and chronic oesophagitis after gastro-oesophageal reflux disease (GORD).13 Based on a recent systemic review of population-based studies, conducted principally in Europe and North America, the prevalence of EoE in adults was approximately 42 per 100,000, which is consistent with EoE being classified as a rare or ‘orphan’ disease (i.e. less than 50 per 100,000 or less 1 in 2000). However, there has been a steady increase in both the incidence and prevalence of EoE documented over the last several years. This might be related to greater awareness of the disease, in addition to the establishment of specific diagnostic guidance, although the actual augmentation in the epidemiology of this disease cannot be discounted.14,15
Eosinophilic oesophagitis can develop at any age, however, onset is principally during late adolescence and between the ages of 30 and 50, with males disproportionally afflicted.13,16,17 EoE is a common cause of food bolus obstruction (FBO), however, is it possible that EoE is not diagnosed in many patients presenting with a FBO due to endoscopies not being performed, or if they are, oesophageal biopsies are not collected.18,19
EoE is characterised clinically by symptoms of oesophageal dysfunction and histologically by eosinophilic infiltration of the oesophageal mucosa. Left untreated, the disorder is associated with the risk of inflammation and progressive fibrosis and development of a small-calibre oesophagus. The primary symptom of EoE is dysphagia: patients experience difficulty swallowing solid foods such as bread or meat and are at risk of food bolus impaction, in some cases requiring extraction by endoscopy in an emergency department. About half of all patients also report retrosternal pain. For some patients, the dysphagia becomes severe and may require dilation with an endoscopy to effectively manage these symptoms. Other symptoms include heartburn and regurgitation (during or immediately after eating).

-
ADULTS
- Dysphagia (difficulty swallowing)
- Odynophagia (pain with swallowing)
- Bolus obstruction
- Chest pain (during or immediately after eating)
- Heartburn
- Regurgitation (during or immediately after eating)
Therefore, investigation of the patient’s medical history should specifically focus on difficulty swallowing and use adaptive behaviours taken to counteract these difficulties when eating solid foods. A discussion around mental health would also be an important consideration. This allows adequate assessment of the severity of symptoms and the associated disease burden on the patient. Furthermore, this provides a baseline to reference when evaluating the impact of a therapeutic intervention in the patient, including whether any of these adaptive behaviours have changed with this treatment. The latter could be particularly relevant if the patient still has some symptoms of oesophageal dysfunction after the initiation of the treatment, however, they are no longer avoiding certain foods or needing to drink a lot of liquids while eating, and/or eating slowly, etc.



Eosinophilic oesophagitis (EoE) is a chronic, progressive inflammatory disease. Left untreated, the risk of oesophageal remodelling, leading to primary fibrostenotic phenotype with one of more strictures (e.g. small calibre oesophagus) increases over time; see Fig. 6 & 7). This is associated with worsening symptoms and the risk for the requirement for endoscopic rescue for small dilation and/or food bolus obstruction.23,24 The progressive nature of the disease is also demonstrated by manometry studies that reveal progressive loss of oesophageal motility over the duration of the disease.25


- Attwood SE. Overview of eosinophilic oesophagitis. Br J Hosp Med (Lond). 2019; 80(3):132–138.
- Gonsalves NP, and Aceves SS. Diagnosis and treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2020; 145(1):1–7.
- Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993;38(1):109–16.
- Kim HP and Dellon ES. An Evolving Approach to the Diagnosis of Eosinophilic Esophagitis. Gastroenterol Hepatol (N Y). 2018; 14 (6):358–366.
- Dhar A, Haboubi HN, Attwood SE et al. British Society of Gastroenterology (BSG) and British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN) joint consensus guidelines on the diagnosis and management of eosinophilic oesophagitis in children and adults. Gut. 2022; 71(8):1459–1487.
- González-Cervera J, Arias Á, Redondo-González O, Cano-Mollinedo MM, Terreehorst I, Lucendo AJ. Association between atopic manifestations and eosinophilic oesophagitis: a systematic review and meta-analysis. Ann Allergy Asthma Immunol 2017; 118(5):582– 90.e2.
- Lucendo AJ, Arias Á, Tenías JM. Relation between eosinophilic oesophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol 2014; 113(6):624–9.
- Almansa C, Krishna M, Buchner AM, Ghabril MS, Talley N, DeVault KR et al. Seasonal distribution in newly diagnosed cases of eosinophilic oesophagitis in adults. Am J Gastroenterol 2009; 104(4):828–33.
- Molina-Infante J, Arias Á, Alcedo J, García-Romero R, Casabona-Frances S, Prieto-García A et al. Step-up empiric elimination diet for pediatric and adult eosinophilic oesophagitis: The 2-4-6 study. J Allergy Clin Immunol 2018; 141(4):1365–72.
- Miehlke S, Alpan O, Schröder S, Straumann A. Induction of eosinophilic oesophagitis by sublingual pollen immunotherapy. Case Rep Gastroenterol 2013; 7(3):363–8.
- Dellon ES and Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology. 2018; 154(2):319–332.e3
- O’Shea KM, Aceves SS, Dellon ES, Gupta SK, Spergel JM, Furuta GT et al. Pathophysiology of eosinophilic oesophagitis. Gastroenterology 2018; 154(2):333–45.
- Arias Á, Pérez-Martínez I, Tenías JM, Lucendo AJ. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic ooesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther 2016; 43(1):3–15.
- Navarro P, Arias Á, Arias-Gonzalez L, Laserna-Mendieta EJ, Ruiz-Ponce M, Lucendo AJ. Systematic review with meta-analysis: the growing incidence and prevalence of eosinophilic ooesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther 2019; 49(9):1116–25.
- orpha.net. Orphan drugs in Australia. Available at www.orpha.net/consor/cgi-bin/Education_AboutOrphanDrugs.php?lng=EN&stapage=ST_EDUCATION_EDUCATION_ABOUTORPHANDRUGS_AUS. Accessed August 2022.
- Hruz P, Straumann A, Bussmann C, Heer P, Simon HU, Zwahlen M et al. Escalating incidence of eosinophilic oesophagitis: a 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol 2011; 128(6):1349–50.e5.
- Simon D, Cianferoni A, Spergel JM, Aceves S, Holbreich M, Venter C et al. Eosinophilic oesophagitis is characterized by a non-IgE mediated food hypersensitivity. Allergy 2016; 71(5):611–20.
- Sharma A, Philpott H. Frontline Gastroenterology 2019;0:1–2. doi:10.1136/flgastro-2019-101245.
- Cook D. et al Intern. Med. J. 49 (2019) 1032–1034)
- Miehlke S. Clinical features of Eosinophilic oesophagitis in children and adults. Best Pract Res Clin Gastroenterol 2015; 29(5):739–48.
- Taft TH, Guadagnoli L and Edlynn. Anxiety and depression in Eosinophilic Esophagitis: A scoping review and recommendations for future research. J Asthma Allergy 2019:12; 389–399.
- Schoepfer AM, Straumann A, Pancazk R et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014; 147(6):1255–66.e21.
- Lucendo AJ, Molina-Infante J, Arias Á, von Arnim U, Bredenoord AJ, Bussmann C et al. Guidelines on eosinophilic oesophagitis: evidence based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J 2017; 5(3):335–58.
- Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon HU et al. Delay in diagnosis of eosinophilic oesophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013; 145(6):1230–6.e1–2.
- van Rhijn BD, Oors JM, Smout AJ, Bredenoord AJ. Prevalence of esophageal motility abnormalities increases with longer disease duration in adult patients with eosinophilic oesophagitis. Neurogastroenterol Motil 2014; 26(9):1349–55.